In the race to secure affordable, sustainable alternatives to lithium-ion batteries, sodium-ion technology has increasingly emerged as a viable contender.
But material instability—particularly in sodium manganese oxide cathodes—has hampered its progress. Now, a team of Japanese researchers has identified a key material-level solution that could alter the trajectory of sodium-ion development: copper doping to eliminate stacking faults in β-NaMnO₂.
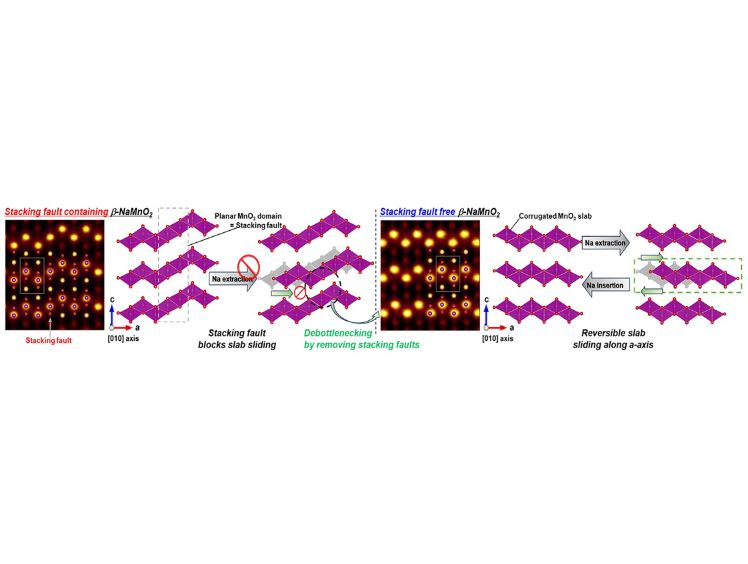
β-phase NaMnO₂ has long been recognized as a promising cathode material due to its high theoretical capacity and favorable voltage profile. However, the structure is prone to stacking faults (SFs)—crystallographic disruptions that mimic the α-phase and deteriorate electrode performance over repeated cycling. These faults not only reduce capacity retention but also obscure the understanding of the β-phase’s complex structural dynamics.
The central challenge lies in controlling these SFs during synthesis. β-NaMnO₂ is typically produced at high temperatures, which can result in sodium-deficient phases and introduce non-equilibrium defects. Until now, methods to mitigate SFs remained largely ineffective or resulted in trade-offs in electrochemical performance.
Copper Doping as Structural Stabilizer
A new study led by Professor Shinichi Komaba at the Tokyo University of Science presents a systematic approach to address these structural instabilities. The researchers doped β-NaMnO₂ with varying levels of copper, producing a series of NaMn₁₋ₓCuₓO₂ samples. The objective was to evaluate how Cu incorporation affects SF formation and cycling performance in sodium-ion half cells.
X-ray diffraction analysis revealed a clear trend: higher Cu content correlated with fewer stacking faults. Notably, the NMCO-12 sample, with 12% Cu doping, exhibited just 0.3% SF concentration—a dramatic reduction compared to the 4.4% seen in lightly doped NMCO-05. Electrochemical tests showed that while undoped samples degraded within 30 cycles, the NMCO-12 maintained its capacity over 150 cycles without noticeable loss.
This near-elimination of SFs offered a rare opportunity to study the intrinsic behavior of β-NaMnO₂ during Na insertion and extraction. In situ and ex situ XRD measurements, supported by density functional theory (DFT) calculations, revealed a distinct mechanism: drastic gliding of MnO₂ layers unique to the β-phase. These shifts were previously masked by structural disorder, making this finding not only a performance milestone but also a breakthrough in materials science.
From a cost perspective, manganese and sodium are far more abundant and geographically secure than lithium and cobalt. The use of Cu as a stabilizing dopant—an element already widely available—further enhances the scalability of this solution. Applications could extend from consumer electronics to electric vehicles and grid-scale storage, where cost and longevity are paramount.
Stay updated on the latest in energy! Follow us on LinkedIn, Facebook, and X for real-time news and insights. Don’t miss out on exclusive interviews and webinars—subscribe to our YouTube channel today! Join our community and be part of the conversation shaping the future of energy.