A recent patent filing reveals an advanced electrolytic reaction system designed to enhance the efficiency of producing gaseous hydrogen and oxygen.
Submitted on August 9, 2024, this patent, developed by unnamed inventors, introduces a specialized electrode design within an electrolytic reaction chamber.
Features and Improvements
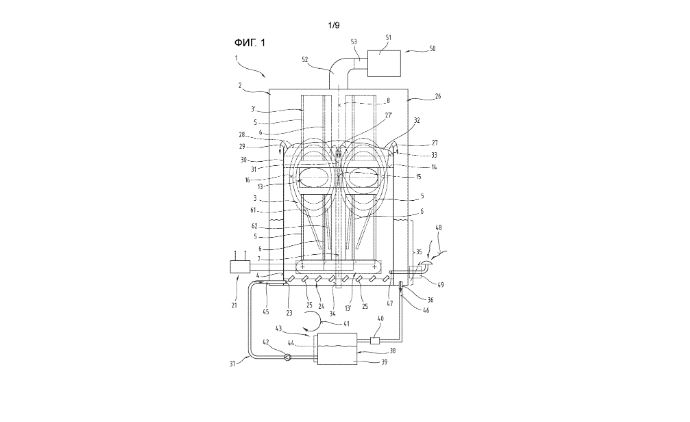
The patented system stands out due to its innovative electrode configuration. It utilizes a plurality of anode and cathode electrodes arranged coaxially in tubular forms. Key to its effectiveness is the spacing between the tubular electrodes, which forms multiple flow channels for the electrolyte. These flow channels span from the electrolyte inlet at the first axial end to the outlet at the second axial end of the electrode device.
What sets this system apart is the deliberate variation in the size of the flow channels. The first flow area is relatively larger to facilitate initial electrolyte movement, while a smaller second flow area near the second axial end ensures controlled flow, optimizing electrolytic reactions. The larger gap at the vertical first axial end gradually narrows towards the second axial end, enhancing the flow dynamics and reaction efficiency significantly.
Potential Applications
This advanced electrolytic system has broad potential applications in industries focused on hydrogen production. Firstly, it can play a crucial role in generating hydrogen for fuel cells, critical for sustainable energy solutions. Additionally, it can be employed in industrial processes requiring high-purity hydrogen and oxygen, such as chemical synthesis and metal refining.
Technical Specifications and Methodologies
The patent elaborates on several technical specifications. The reaction chamber is tailored to accommodate multiple anode and cathode electrodes, and the critical gaps between the electrodes are engineered to optimize flow. The flow channel’s design ensures that the flow rate and electrolyte distribution are precisely managed to enhance the overall efficiency of the electrolytic reaction.